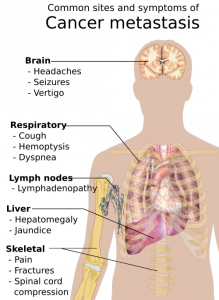
Cancer is a fatal illness with poor prognosis and survival rate, especially when it has progressed to a metastatic state. Scientists have not yet been able to decipher why patients become resistant to chemotherapeutic drugs and therapies. To address this objective, researchers worked diligently at the Rogel Cancer Center—it is affiliated to the University of Michigan. They made an important breakthrough in the year 2003. The lead supervisor was Dr S. Wicha, MD for the team of researchers. They found that there are cancer stem cells that act like a fuel within a tumor. Although this group of cells is immensely small, they are the ones that trigger the growth and metastasis of cancer. The simple strategy was then to simply kill the group of cancer stem cells, and the long lost battle against cancer could be defeated easily. But, is this so easy to sound hopeful for cancer patients? Not really, cancer is such a condition that can relapse and attack patients even after they have been cured temporarily.
Currently, there has been an important discovery: cancer stem cells do not really exist in ONLY a single state but they are exhibited in different states; they are immensely plastic in nature. This implies that different forms can be easily adopted by cancer stem cells. They could be in a dormant state for some point of time and then easily bounce back into uncontrolled growth, leading to formation of tumor. Multiplication and spreading, the two characteristic features of cancer stem cells, have been attributed to its most important property: plasticity.
Presently, patients are treated with targeted therapies for combating cancer. Although these therapies are effective, they have been successful in destroying tumor cells only for a certain period of time. There are many cases in which patients develop resistance to these targeted therapies. What is the cause of drug resistance in cancer patients? Most scientists believe that drug resistance is triggered once again by cancer stem cells. Because cancer stem cells have high plasticity, they change their form completed when subjected to targeted therapies. The resultant effect is that cancer stem cells are completely unrecognizable to these therapies following change of form. The patient thus develops resistance to therapies and the patients’ condition deteriorates consistently.
The conclusion: multiple stem cell therapies must be developed to effectively combat every form of cancer stem cell. This is a humungous task to achieve according to scientists at the Rogel Cancer Center. Cell metabolism is the key feature that controls the plasticity of cancer stem cells. How do we eliminate the plasticity of cancer stem cells? Well, all we need to do is to target the metabolism of cancer stem cells. In other words, cancer stem cells can be effectively attacked by destroying cell metabolism.
Mitochondria are cell organelles that supply energy to cells, irrespective of its kind. This includes cancer stem cells. Mitochondria are organelles that perform cellular respiration, depending completely on the supply of oxygen. Cells derive energy from mitochondria, which converts sugar or glucose molecules into energy with the help of cellular oxygen. Cancer stem cells are very unique due to its plasticity. When they are in the dormant state, they derive energy from glucose molecule. When they grow in a proliferative state, cancer stem cells depend completely on oxygen.
Given the mechanism of deriving energy for sustenance and proliferation, researchers attacked both forms of cell metabolism observed in cancer stem cells. They used a drug that is conventionally used for treating arthritis. This drug can effectively block the functioning of mitochondria in cancer stem cells. The levels of cellular glucose were further manipulated to obstruct the pathway of energy. They performed this experiment on cancer-stricken mice. To their surprise, they had effectively knocked off all the cancer stem cells from the mice. This is an important breakthrough in cancer research, and the findings of this study have attracted a lot of attention. The complete experiment has been published in Cell Metabolism, a peer-reviewed SCI journal.
The general public may wonder why this study is so path-breaking and innovative in nature. Well, the conventional cancer therapy makes use of highly toxic chemicals to destroy cells in a tumor. Here, researchers adopted a completely different pathway to control the explosion of cancer stem cells: they destroyed the cell metabolism associated with the proliferation of tumor cells. According to the lead researcher Dr. Wicha, further studies must be conducted to understand how metabolism controls the efficacy of human immune system. This could open a new chapter in cancer research: scientists could then focus their efforts on developing novel combinatorial techniques for cancer treatment. These techniques must aim at effectively combining existing immunotherapies with anti-stem cell therapies. The concept is refreshing and offering new hope; however, extensive clinical trials must be conducted to validate results.


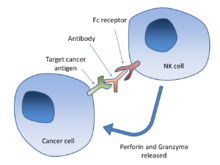
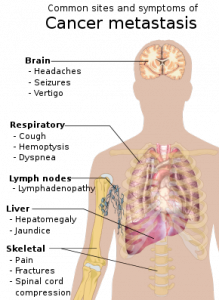
 The drug Lutathera (lutetium Lu 177 dotatate) was approved by US FDA for treating neuroendocrine tumors that originate in the gastrointestinal tract and pancreas (GEP-NETs). This is an important breakthrough as it is the first radioactive drug to have been approved by the FDA. It is now a novel treatment for GEP-NETs.
The drug Lutathera (lutetium Lu 177 dotatate) was approved by US FDA for treating neuroendocrine tumors that originate in the gastrointestinal tract and pancreas (GEP-NETs). This is an important breakthrough as it is the first radioactive drug to have been approved by the FDA. It is now a novel treatment for GEP-NETs.