The UC Davis Comprehensive Cancer Center has conducted an in-depth research study. The focus was to activate programmed death of cancer cells. A crucial epitope has been identified on the CD95 receptor, and it triggers the death of cells. An epitope is a section of protein that activates the larger protein. As cell death can now be programmed, cancer treatment methods have become effective.
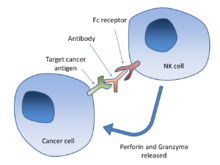
In molecular biology, CD95 receptors are known as Fas and death receptors. These receptors are proteins present in cell membrane. They evoke self-destruction of cells by releasing a signal. This happens when CD95 receptors are activated. By modulating the activity of Fas, researchers extended its benefits to CAR T-cell therapy. This was effective in destroying solid tumours of ovarian cancer.
Managing cancer with better therapies
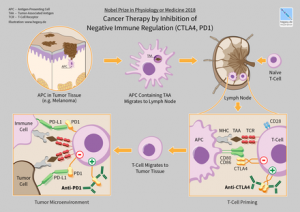
The conventional method of treating cancer includes chemotherapy, radiotherapy, and surgery. In cases where cancer is diagnosed at an initial stage, these methods are effective. However, cancer cases may relapse, especially when they are therapy-resistant. Recently, CAR T-cell immunotherapy and an immune checkpoint receptor molecule have shown to activate antibodies, and thus they are promising candidates that destroy the cycle of cancerous growth.
These immunotherapeutic agents are effective against only few types of cancer cells, such as ovarian cancer, breast cancer, lung cancer, and pancreatic cancer. In CAR T-cell therapy, researchers engineer the specific type of immune cells, that is, T cells. They graft these cells on a specific antibody that targets specific tumours. The grafted T cells are quite effective in battling leukaemia and other types of blood cancer.
The engineered T cells have not been effective in combating solid tumours; the microenvironment of these tumours drives off T cells and other immune cells. Thus, they cannot provide a therapeutic effect to solid tumours. Although the immune receptor activates antibodies, the T cells cannot infiltrate without additional spaces.
The activity of death receptors
Now, let us understand the activity of death receptors. Through targeted therapy, we can trigger them into programming cell death of tumours. Thus, chemotherapeutic drugs should be such that they induce the activity of death receptors. Many pharmaceutical companies have been slightly successful in targeting the death receptor-5. But the clinical trials of Fas agonists have failed.
Developing the right target
The activity of immune cells is effectively regulated by Fas. However, researchers have proposed that cancer cells can be targeted selectively if they identify the correct epitope. After identifying the targeted epitope, researchers of this study have designed a new type of antibodies. These antibodies show selectivity while binding and activating Fas. With this strategy, specific tumor cells can be destroyed.