Plaques developed in the brain can be eliminated with a low-dose aspirin, which is an effective drug that suppresses the progression of Alzheimer’s disease. The drug aspirin is very effective in protecting the memory of patients. These are the latest findings reported by neurologists at the Rush University Medical Center. The results of this study were published in the Journal of Neuroscience.
Our study is path-breaking and novel in the sense that aspirin is one of the most commonly used medication for various illnesses. More than 1 out of 10 Americans was diagnosed with Alzheimer’s disease, which is a progressive form of dementia. Very few drugs have been approved by the FDA for the treatment of Alzheimer’s-related complications, such as dementia. Presently, only temporary relief is provided by these medications.
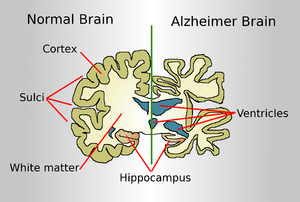
Researchers still do not know the exact cause of Alzheimer’s disease; however, researchers know the cause of dementia and memory loss, which is associated with the faulty disposal of amyloid beta. Amyloid beta is the most toxic protein to have been developed in the human brain. Researchers believe that the most important strategy for eliminating the progression of Alzheimer’s illness would be the activation of cellular machinery. Waste can be removed from the human brain with this machinery.
Amyloid plaques are clumps formed by the toxic protein amyloid beta. The connection between nerve cells would be harmed by amyloid plaques. Such a development is one of the major signs of Alzheimer’s illness. There seems to be a link between the reduced risk of developing Alzheimer’s disease and the consumption of aspirin. The most important component of animal cells, the lysosomes, is very useful in clearing cellular debris. In mice, lysosomes could be stimulated with aspirin. Aspirin is the component that decreases amyloid plaque.
The incidence, progression, and development of Alzheimer’s disease could be stopped by elucidating the development of amyloid plaques. To regulate the removal of waste products from the human body, a protein named TFEB. Aspirin was administered orally to mice, which were genetically modified to develop the pathology of Alzheimer’s disease.
To determine the parts of brain most affected by Alzheimer’s disease, we determined the amount of amyloid plaque in these subjects. In mice, the functions of aspirin medications are as follows: i) to augment the expression of TFEB, ii) stimulate the expression of lysosomes, and iii) decrease the pathology of amyloid plaque.
Aspirin is the most widely used medication for pain relief; moreover, it is also used extensively for the treatment of cardiovascular diseases. The findings of these research studies must be validated further. Aspirin could be soon considered as a therapeutic drug for the treatment of Alzheimer’s illness and other diseases related to dementia.

